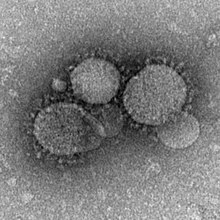
Middle East respiratory syndrome (MERS) is a viral respiratory infection caused by the newly identified MERS-coronavirus (MERS-CoV). MERS-CoV is a betacoronavirus derived from bats. Camels have been shown to have antibodies to MERS-CoV, but the exact source of infection in camels has not been identified. A strain of MERS-CoV known as HCoV-EMC/2012 found in the first patient in London in 2012 was found to have a 100% match to Egyptian tomb bats.
Signs and symptoms

Early reports compared the virus to severe acute respiratory syndrome (SARS), and it has been referred to as Saudi Arabia's SARS-like virus. The first patient, in June 2012, had a "seven-day history of fever, cough, expectoration, and shortness of breath." One review of 47 laboratory confirmed cases in Saudi Arabia gave the most common presenting symptoms as fever in 98%, cough in 83%, shortness of breath in 72% and myalgia in 32% of patients. There were also frequent gastrointestinal symptoms with diarrhea in 26%, vomiting in 21%, abdominal pain in 17% of patients. 72% of patients required mechanical ventilation. There were also 3.3 males for every female. One study of a hospital based outbreak of MERS had an estimated incubation period of 5.5 days (95% confidence interval 1.9 to 14.7 days). MERS can range from asymptomatic disease to severe pneumonia leading to the acute respiratory distress syndrome. Renal failure, disseminated intravascular coagulation (DIC) and pericarditis have also been reported.
Diagnosis
The World Health Organization (WHO) publishes laboratory guidance, surveillance and investigation, case definition, and other materials on its website. The current interim case definition is that a confirmed case is identified in a person with a positive lab test by "molecular diagnostics including either a positive PCR on at least two specific genomic targets or a single positive target with sequencing on a second."
History
According to the WHO, a probable case is
- a person with a febrile acute respiratory illness with clinical, radiological, or histopathological evidence of pulmonary parenchymal disease (e.g. pneumonia or acute respiratory distress syndrome)
and
testing for MERS-CoV is unavailable or negative on a single inadequate specimen
and
the patient has a direct epidemiologic link with a confirmed MERS-CoV case. - A person with a acute febrile respiratory illness with clinical, radiological, or histopathological evidence of pulmonary parenchymal disease (e.g. pneumonia or acute respiratory distress Syndrome)
and
an inconclusive MERS-CoV laboratory test (that is, a positive screening test without confirmation)
and
a resident of or traveler to Middle Eastern countries where MERS-CoV virus is believed to be circulating in the 14 days before onset of illness. - A person with an acute febrile respiratory illness of any severity
and
an inconclusive MERS-CoV laboratory test (that is, a positive screening test without confirmation)
and
the patient has a direct epidemiologic-link with a confirmed MERS-CoV case.
In the United States, the Centers for Disease Control and Prevention (CDC) recommend investigating any person with:
- fever (≥38 °C, 100.4 °F) and pneumonia or acute respiratory distress syndrome (based on clinical or radiological evidence)
and either:
-
- a history of travel from countries in or near the Arabian Peninsula within 14 days before symptom onset, or
- close contact with a symptomatic traveler who developed fever and acute respiratory illness (not necessarily pneumonia) within 14 days after traveling from countries in or near the Arabian Peninsula or
- a member of a cluster of patients with severe acute respiratory illness (e.g. fever and pneumonia requiring hospitalization) of unknown etiology in which MERS-CoV is being evaluated, in consultation with state and local health departments.
or
- close contact with a confirmed or probable case of MERS while the case was ill and
- fever (>100 °F) or symptoms of respiratory illness within 14 days following the close contact.
Radiology
Chest X-ray findings tend to show bilateral patchy infiltrates consistent with viral pneumonitis and ARDS. Lower lobes tend to be more involved. CT scans show interstitial infiltrates.
Laboratory testing
MERS cases have been reported to have leucopenia, and in particular lymphopenia.
For PCR testing the WHO recommends obtaining samples from the lower respiratory tract via bronchoalveolar lavage (BAL), sputum sample or tracheal aspirate as these have the highest viral loads. There have also been studies utilizing upper respiratory sampling via nasopharyngeal swab.
Several highly sensitive, confirmatory real-time RT-PCR assays exist for rapid identification of MERS-CoV from patient-derived samples. These assays attempt to amplify upE (targets elements upstream of the E gene), open reading frame 1B (targets the ORF1b gene) and open reading frame 1A (targets the ORF1a gene). The WHO recommends the upE target for screening assays as it is highly sensitive. In addition, hemi-nested sequencing amplicons targeting RdRp (present in all coronaviruses) and nucleocapsid (N) gene (specific to MERS-CoV) fragments can be generated for confirmation via sequencing. Reports of potential polymorphisms in the N gene between isolates highlight the necessity for sequence-based characterization.
The WHO recommended testing algorithm is to start with an upE RT-PCR and if positive confirm with ORF 1A assay or RdRp or N gene sequence assay for confirmation. If both an upE and secondary assay are positive it is considered a confirmed case.
Protocols for biologically safe immunofluorescence assays (IFA) have also been developed; however, antibodies against betacoronaviruses are known to cross-react within the genus. This effectively limits their use to confirmatory applications. A more specific protein-microarray based assay has also been developed that did not show any cross-reactivity against population samples and serum known to be positive for other betacoronaviruses. Due to the limited validation done so far with serological assays, WHO guidance is that "cases where the testing laboratory has reported positive serological test results in the absence of PCR testing or sequencing, are considered probable cases of MERS-CoV infection, if they meet the other conditions of that case definition."
Treatment

Although MERS-CoV has been shown to antagonize endogenous IFN production, treatment with exogenous types I and IIIIFN (IFN-α and IFN-λ, respectively) have effectively reduced viral replication in vitro. When rhesus macaques were given interferon-α2b and ribavirin and exposed to MERS, they developed less pneumonia than control animals. 5 critically ill patients with MERS in Saudi Arabia with ARDS and on ventilators were given interferon-α2b and ribavirin but all ended up dying of the disease. The treatment was started late in their disease (a mean of 19 days after hospital admission) and they had already failed trials of steroids so it remains to be seen whether it may have benefit earlier in the course of disease. Another proposed therapy is inhibition of viral protease. Researchers are investigating a number of ways to combat the outbreak of Middle East respiratory syndrome coronavirus, including using interferon, chloroquine, chlorpromazine, loperamide, and lopinavir.
Transmission

Camels
A study performed between 2010 and 2013, in which the incidence of MERS was evaluated in 310 dromedary camels, revealed high titers of neutralizing antibodies to MERS-CoV in the blood serum of these animals. A further study sequenced MERS-CoV from nasal swabs of dromedary camels in Saudi Arabia and found they had sequences identical to previously sequenced human isolates. Some individual camels were also found to have more than one genomic variant in their nasopharynx. There is also a report of a Saudi Arabian man who became ill seven days after applying topical medicine to the noses of several sick camels and later he and one of the camels were found to have identical strains of MERS-CoV. It is still unclear how the virus is transmitted from camels to humans. The World Health Organization advises avoiding contact with camels and to eat only fully cooked camel meat, pasturized camel milk, and to avoid drinking camel urine. Camel urine is considered a medicine for various illnesses in the Middle East. The Saudi Ministry of Agriculture has advised people to avoid contact with camels or wear breathing masks when around them. In response "some people have refused to listen to the government's advice" and kiss their camels in defiance of their government's advice.
Person to person
There has been evidence of limited, but not sustained spread of MERS-CoV from person to person, both in households as well as in health care settings like hospitals. Most transmission has occurred "in the circumstances of close contact with severely ill patients in healthcare or household settings" and there is no evidence of transmission from asymptomatic cases. Cluster sizes have ranged from 1 to 26 people, with an average of 2.7.
Prevention
Infection control
While the mechanism of spread of MERS-CoV is currently not known, based on experience with prior coronaviruses, such as SARS, the WHO currently recommends that all individuals coming into contact with MERS suspects should (in addition to standard precautions):
- Wear a medical mask
- Wear eye protection (i.e. goggles or a face shield)
- Wear a clean, non sterile, long sleeved gown; and gloves (some procedures may require sterile gloves)
- Perform hand hygiene before and after contact with the patient and his or her surroundings and immediately after removal of personal protective equipment
For procedures which carry a risk of aerosolization, such as intubation, the WHO recommends that care providers also:
- Wear a particulate respirator and, when putting on a disposable particulate respirator, always check the seal
- Wear eye protection (i.e. goggles or a face shield)
- Wear a clean, non-sterile, long-sleeved gown and gloves (some of these procedures require sterile gloves)
- Wear an impermeable apron for some procedures with expected high fluid volumes that might penetrate the gown
- Perform procedures in an adequately ventilated room; i.e. minimum of 6 to 12 air changes per hour in facilities with a mechanically ventilated room and at least 60 liters/second/patient in facilities with natural ventilation
- Limit the number of persons present in the room to the absolute minimum required for the patient’s care and support
- Perform hand hygiene before and after contact with the patient and his or her surroundings and after PPE removal.
The duration of infectivity is also unknown so it is unclear how long patients must be isolated, but current recommendations are for 24 hours after resolution of symptoms. In the SARS outbreak the virus was not cultured from patients after the resolution of their symptoms.
It is believed that the existing SARS research may provide a useful template for developing vaccines and therapeutics against a MERS-CoV infection. Vaccine candidates are currently awaiting clinical trials.
History
Collaborative efforts were used in the identification of the MERS-CoV. Egyptian virologist Dr. Ali Mohamed Zaki isolated and identified a previously unknown coronavirus from the lungs of a 60-year-old Saudi Arabian man with pneumonia and acute renal failure. After routine diagnostics failed to identify the causative agent, Zaki contacted Ron Fouchier, a leading virologist at the Erasmus Medical Center (EMC) in Rotterdam, the Netherlands, for advice. Fouchier sequenced the virus from a sample sent by Zaki.
Fouchier used a broad-spectrum "pan-coronavirus" real-time polymerase chain reaction (RT-PCR) method to test for distinguishing features of a number of known coronaviruses (such as OC43, 229R, NL63, and SARS-CoV), as well as for RNA-dependent RNA polymerase (RdRp), a gene conserved in all coronaviruses known to infect humans. While the screens for known coronaviruses were all negative, the RdRp screen was positive.
On 15 September 2012, Dr. Zaki's findings were posted on ProMED-mail, the Program for Monitoring Emerging Diseases, a public health on-line forum.
The UK Health Protection Agency (HPA) confirmed the diagnosis of severe respiratory illness associated with a new type of coronavirus in a second patient, a 49-year-old Qatari man who had recently been flown into the UK. He died from an acute, serious respiratory illness in a London hospital. In September 2012, the United Kingdom's Health Protection Agency (HPA) named it the London1 novel CoV/2012 and produced the virus' preliminary phylogenetic tree, the genetic sequence of the virus based on the virus's RNA obtained from the Qatari case.
On 25 September 2012, the WHO announced that it was "engaged in further characterizing the novel coronavirus" and that it had "immediately alerted all its Member States about the virus and has been leading the coordination and providing guidance to health authorities and technical health agencies." The Erasmus Medical Center in Rotterdam "tested, sequenced and identified" a sample provided to EMC virologist Ron Fouchier by Ali Mohamed Zaki in November 2012.
On 8 November 2012 in an article published in the New England Journal of Medicine, Dr. Zaki and co-authors from the Erasmus Medical Center published more details, including a tentative name, Human Coronavirus-Erasmus Medical Center (HCoV-EMC), the virus’s genetic makeup, and closest relatives (including SARs).
In May 2013, the Coronavirus Study Group of the International Committee on Taxonomy of Viruses adopted the official designation, the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), which was adopted by WHO to "provide uniformity and facilitate communication about the disease." Prior to the designation, WHO had used the non-specific designation 'Novel coronavirus 2012' or simply 'the novel coronavirus'.
In April 2014 MERS emerged in the Philippines with a suspected case of a home bound OFW (overseas foreign worker). Several suspected cases involving individuals who were on the same flight as the initial suspected case are being tracked but are believe to have dispersed throughout the country. Another suspected MERS-involved death in Sultan Kudarat province caused the DOH to put out an alert.
MERS was also implicated in an outbreak in April 2014 in Saudi Arabia, where MERS has infected 688 people and 282 MERS-related deaths have been reported since 2012. In response to newly reported cases and deaths, and the resignation of four doctors at Jeddah’s King Fahd Hospital who refused to treat MERS patients for fear of infection, the government has removed the Minister of Health and set up three MERS treatment centers. 18 more cases were reported in early May.
On 2 May 2014, the United States Centers for Disease Control confirmed the first diagnosis of MERS in the United States in Indiana. The man diagnosed was a health care worker who had been in Saudi Arabia a week earlier, and was reported to be in good condition. A second patient who also traveled from Saudi Arabia was reported in Orlando, Florida on 12 May 2014. On 14 May 2014, officials in the Netherlands reported the first case has appeared. On Saturday, May 17, 2014, a man from Illinois who was a business associate of the first U.S. case (he had met and shook hands with the Indiana health care worker) tested positive for the MERS coronavirus, but has not, as of yet, displayed symptoms (others are probably also, at least temporarily if not permanently, non-symptomatic carriers). The CDC's Dr. David Swerdlow, who is leading the agency's response, said the man, who feels well and has not yet sought and does not yet need medical care, has not been deemed an official case yet and prevention guidelines have not changed. Laboratory tests showed evidence of past infection in his blood.
In June 2014, Saudi Arabia announced 113 previously unreported cases of MERS, revising the death toll to 282, and fired its minister of health.
References








I'm 61 years old, I contracted hpv in 2011' I has be taking lot treatment for it and some months ago the wart stated coming out seriously, I used lot recommendation because there was lot warts around my anus and was so embarrassed. but today I'm totally happy I got the virus eliminated by using natural treatment from Dr Onokun herbal center after his treatment I got cured. all the warts went away' seriously believed Dr Onokun he have the cure for human papillomavirus because he has eliminated hpv been in my body since 2011, Dr Onokun make it possible for me. Here is Dr Onokun email to reach him: Dronokunherbalcure@gmail.com he is welled capable of curing terrible diseases.
ReplyDeleteHappiness is all i see now I never thought that I will live on
ReplyDeleteearth before the year runs out. I have been suffering from a
deadly disease (Herpes) for the past 3 years now; I had spent
a lot of money going from one places to another, from
churches to churches, hospitals have been my home every day
residence. Constant checks up have been my hobby not until
this faithful day, I was searching through the internet, I saw a
testimony on how pp him +2348154637647 Dr Lucky, helped
someone in curing his Herpes disease, quickly I copied his
email which is (drluckyherbalcure@gmail.com) just to give
him a test I spoke to him, he asked me to do some certain
things which I did, he told me that he is going to provide the
herbal cure to me, which he did, then he asked me to go for
medical checkup after some days after using the herbal cure, I
was free from the deadly disease, he only asked me to post
the testimony through the whole world, faithfully am doing it
now, please brothers and sisters, he is great, I owe him in
return. if you are having a similar problem just email him on
(drluckyherbalcure@gmail.com) or Call him or WhatsApp him
+2348154637647
Can't still believe that i got cured from Genital Herpes through herbal treatment from Dr LUCKY who I met through the internet, I actually couldn't believe it at first because it sounded impossible to me knowing how far I have gone just to get rid of it. Dr LUCKY send me his medicine which I took as instructed and here I am living a happy life once again, a big thanks to Dr LUCKY , I am sure there are many herbal doctors out there but Dr LUCKY did it for me, contact him on Email him; { drluckyherbalcure@gmail.com }
ReplyDeleteI’m here to testify about what DR. ISIBOR did for me. I have been suffering from (GENITAL HERPES VIRUS) disease for the past 3 years and had constant pain and inching, especially in my private part. During the first year, I had faith in God that i would be cured someday.This disease started circulating all over my body and I have been taking treatment from my doctor, few weeks ago I came across a testimony of Rose Smith on the internet testifying about a Man called DR. ISIBOR on how he cured her from 7 years HSV 2. And she also gave the email address of this man, advise anybody to contact him for help on any kind of diseases that he would be of help, so I emailed him telling him about my (HSV 2) he told me not to worry that I was going to be cured!! Well, I never doubted him I have faith he can cure me too,, DR. ISIBOR prepared and sent me Healing Oil, Soap, roots and herbs which I took. In the first one week, I started experiencing changes all over me, after four weeks of using his Roots/ Herbs, Oil and Soap, I was totally cured. no more inching , pain on me anymore as DR. ISIBOR assured me. After some time I went to my doctor to do another test behold the result came out negative. So friends my advise is if you have such disease or know anyone who suffers from it or any other disease like HPV, HIV, ALS, CANCER etc. you can contact DR. ISIBOR for help via email} {drisiborspellhome@gmail.com} you can also contact him on WhatsApp +2348107855231
ReplyDelete
ReplyDeleteEliminate herpes forever...........................
This herbal Doctor has a cure to herpes virus,
certainly the best online…
Thank you!! for saving my life
Email_______________R.buckler11(@) gmail . com